Deriving Partition Function \(q\) for an Equidistant ladder of Energy Levels
Partition functions give a measure of how many energy levels are available given temperature and molecular conditions.
For an equidistant ladder, the distance between energy levels is a constant \(\varepsilon\)
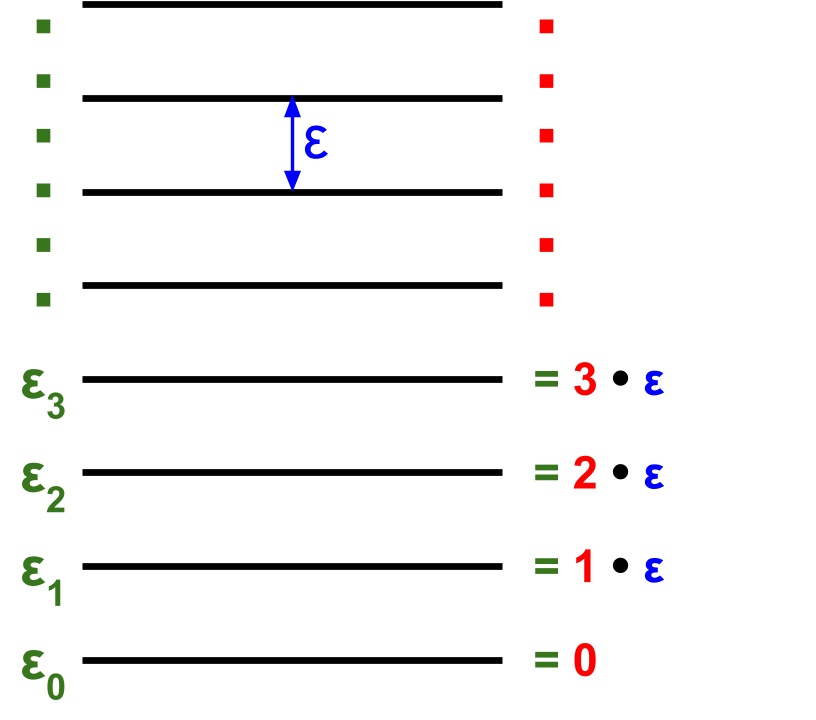
Consider the partition function for a harmonic oscillator
\(\begin{align*} q &= e^{-0 \beta \varepsilon} + e^{-1 \beta \varepsilon} + e^{-2 \beta \varepsilon} + \ldots \\ &= 1 + {\left( e^{- \beta \varepsilon} \right)}^{1} + {\left( e^{- \beta \varepsilon} \right)}^{2} + \ldots \end{align*}\)
If we let \(x = e^{- \beta \varepsilon}\), we can simplify this equation to
\[q = 1 + x + x^2 + \ldots\]This gives us a power series. Assuming \(x = e^{- \beta \varepsilon} < 1\), we can multiply by \(\frac{1-x}{1-x}\) to further simplify this equation
\[\begin{align} q &= \frac{(1-x)}{(1-x)} \left( 1 + x + x^2 + \ldots \right) \\ &= \frac{(1 + x + x^2 + \ldots)-(x + x^2 + x^3 + \ldots)}{(1-x)} \\ &= \frac{1}{(1-x)} \end{align}\]Thus the partition function for an equidistant ladder of energy levels is
\[q = \frac{1}{1-e^{- \beta \varepsilon}}\]